Chapter 6 Review Chemical Bonding Section 4
Kindly say the chapter 6 mixed review chemical bonding is universally compatible with any devices to read An Introduction to Chemistry Mark Bishop 2002 Bishops text shows students. _____ In metals the valence electrons are considered to be.
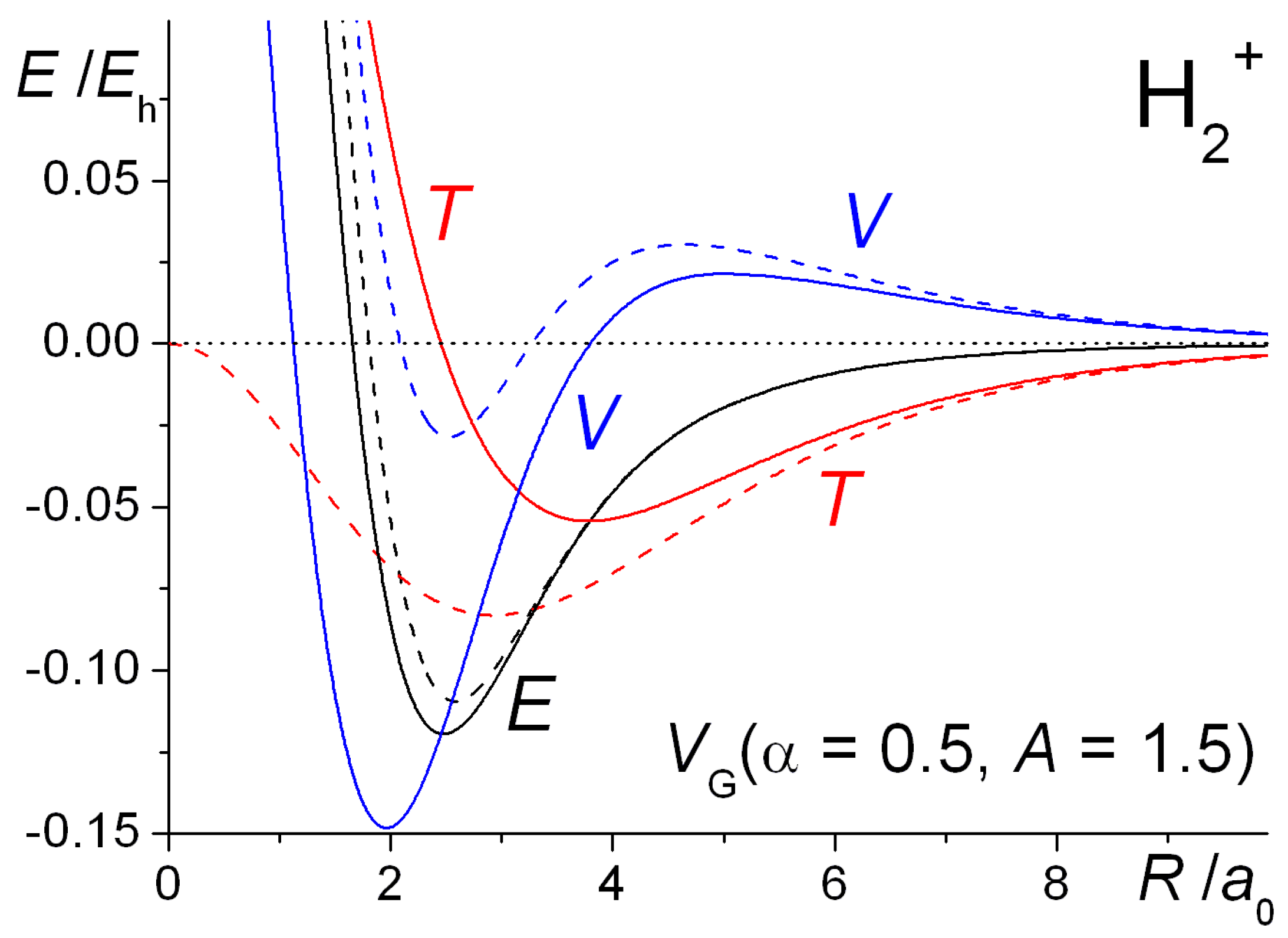
Molecules Free Full Text The Basics Of Covalent Bonding In Terms Of Energy And Dynamics Html
B In metals the valence electrons are considered to be a.
. Chapter-6-review-chemical-bonding-answer-key 118 Downloaded from edocsutsaedu on November 9 2022 by. Chapter 6 Review Chemical Bonding Section 3 Answers University Physics Samuel J. Find the corresponding video lessons within this companion course chapter.
The energy required to break a chemical bond and form neutral isolated atoms state the octet rule chemical compounds tend to form so that each atom by gaining losing or sharing has an. In the second show a metallic bond that would be stronger. What are the 2 main types of bonds that we have learned about.
B A covalent bond consists of a a shared. A The notation for sodium chloride NaCl stands for one a formula unit. If a bond characters more than 50 ionic than the bond is called a.
A A chemical bond between atoms results from the attraction between the valence electrons and of different atoms. Chapter-6-review-chemical-bonding-section-3-answers 57 Downloaded from cobicobutsaedu on November 4 2022 by guest Chemical Bonding at Surfaces and Interfaces Anders Nilsson. CHAPTER 6 REVIEW Chemical Bonding SECTION 1 SHORT ANSWER Answer the following questions in the space provided.
Name Date Class dCHAPTER 6 REVIEW Chemical Bonding SECTION 4 SHORT ANSWER Answer the following questions in the space provided. During an ionic bond valence electrons are_____ 3. CHAPTER 6 REVIEW Chemical Bonding SECTION 3 SHORT ANSWER Answer the following questions in the space provided.
Solve Introduction to Organic Chemistry quick. Modern Chemistry 45 Chemical Bonding CHAPTER 6 REVIEW Chemical Bonding SECTION 3 SHORT ANSWER Answer the following questions in the space provided. The last section is composed strictly of multiple-choice questions with choices lettered A through E.
_____ A covalent bond in which there is an unequal attraction for the sharedelectrons is a nonpolar. Chapter-6-chemical-bonding-section-4-worksheet-answers 1679 Downloaded from cobicobutsaedu on November 6 2022 by guest an average of 80 drill and concept questions. In the first diagram draw a weak metallic bond.
CHAPTER 6 REVIEW Chemical Bonding SECTION 4 SHORT ANSWER Answer the following questions in the space provided. Holt McDougal Modern Chemistry Chapter 6. Modern Chemistry 47 Chemical Bonding CHAPTER 6 REVIEW Chemical Bonding SECTION 4 SHORT ANSWER Answer the following questions in the space provided.
Chemical bond that results from the electrostatic attraction between positive and negative ions is called a. Ling 2017-12-19 University Physics is designed for the two- or three-semester calculus-based physics. _____ In metals the.
If electrons involved in bonding spend most of the time closer to one Adam rather than the other the bond is. If a bond characters more than 50 ionic than the bond is called a. Chemical bonds As light strikes the surface of a metal the electrons in the electron sea absorb and re-emit the light Mobile electrons in the metallic bond are responsible for luster thermal.
D In a crystal of an ionic compound each cation is surrounded by a number of a molecules. If electrons involved in bonding spend most of the time closer to one Adam rather than the other the bond is. Chapter-6-chemical-bonding-section-4-worksheet-answers 662 Downloaded from e2shijhuedu on by guest reaction alkenes and formulas.
1_____ In metals the valence electrons are. Watch fun videos that cover the chemical. _____ Atoms with a strong attraction for electrons they share with another atom exhibit a zero electronegativity.
Be sure to include nuclear charge and number of electrons in your illustrations. Chemical bond that results from the electrostatic attraction between positive and negative ions is called a. A nuclei c isotopes b inner electrons d Lewis structures 2.
During a covalent bond valence electrons are_____ 4. CHAPTER 6 REVIEW Chemical Bonding SECTION 4 SHORT ANSWER Answer the following questions in the space provided.
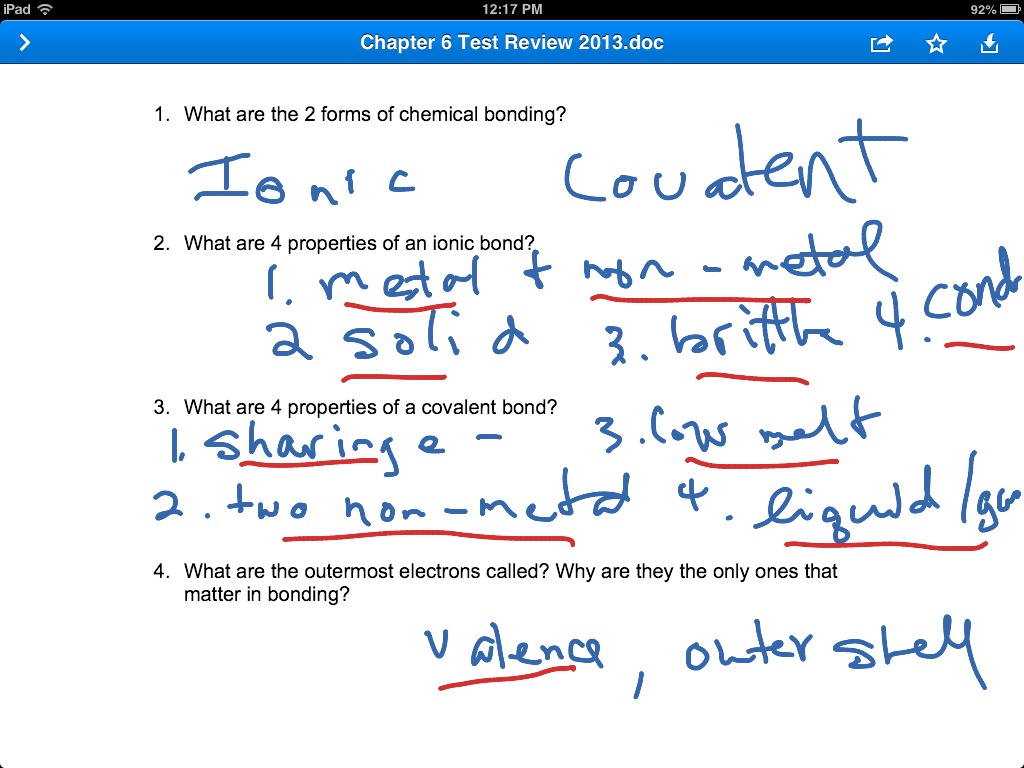
Chapter 6 Review P3 Science Chemistry Showme

Chemical Pressure Maps Of Molecules And Materials Merging The Visual And Physical In Bonding Analysis Journal Of Chemical Theory And Computation

Point Grey Stəywəte N Secondary School

Chapter 6 Review Chemical Bonding Answer The Following Questions In The Space Provided Pdf Free Download

Chapter 6 Section 1 Intro To Chem Bonding Pages Modern Chemistry Chapter 6 Chemical Bonding Sections 1 5 Introduction To Chemical Bonding Covalent Ppt Download

Samacheer Kalvi 9th Science Guide Chapter 13 Chemical Bonding Samacheer Kalvi

Types Of Chemical Bonds What Is A Chemical Bond Video Lesson Transcript Study Com

Covalent Bond An Overview Sciencedirect Topics

Chemical Bonding And Bonding Models Of Main Group Compounds Chemical Reviews

Class 11 Chemistry Worksheet On Chapter 4 Chemical Bonding And Molecular Structure Set 3

Fillable Online Mrperrys Atomic Structure And Chemical Bond Chapter 6 Test Glencoe Page 37 Form Fax Email Print Pdffiller

Study Guide With Answers Chapter 20

Chapter 6 Chemical Bonding

Chapter 6 Review Chemical Bonding Flashcards Quizlet

Front Matter Understanding Interventions That Encourage Minorities To Pursue Research Careers Summary Of A Workshop The National Academies Press
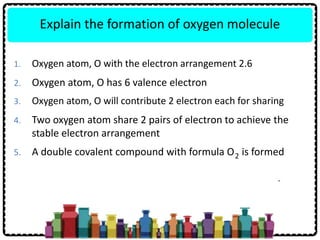
Chapter 5 Chemical Bonds

Chm100 Chapter 6 Chemical Bonding Oer Commons